Groupe Athena Inc.
- GATA

Investment Highlights
Record Revenues continue: $17,961,452 for
3 months ended Dec 2013
Operating income for the quarter: $3,
097,791
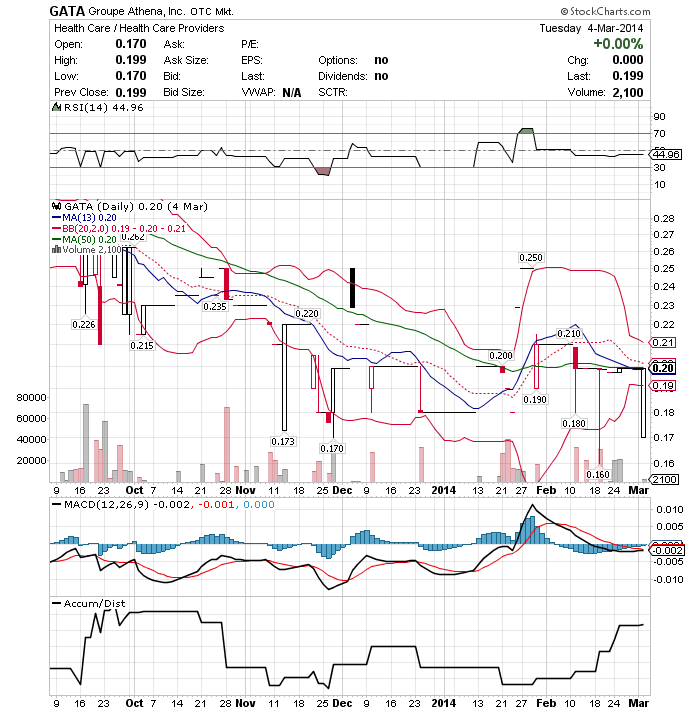
4.4M Float
$56M in revenue for the year ended Jun
2013
About Us
Serving India and South East Asia, Groupe Athena has been serving companies
abroad in obtaining FDA approval for pharmaceuticals, medical products and
devices for export to America. Our goal is to ensure each of our clients
passes FDA inspection by assisting them from concept through development,
with consultation through all the regulatory requirements, filings, and
processes to achieve FDA compliance and approval.
With offices in India and America, the expert consultants of Groupe Athena
are in position to go to target companies in Asia and the regulatory
authorities in the USA directly, consulting with our clients in the East to
serve them completely in providing consultancy services for FDA compliance.
Services include providing technical and regulatory consulting for
biotechnical products, pharmaceuticals [over the counter, prescription and
generics], diagnostics, medical equipment, and devices.
The FDA inspection process is traditionally complicated, especially for
foreign drug and medical companies trying to export to the USA. With broad
spectrum consultancy and assistance through this process, our client base
can count on reduced risk, follow-through on steps necessary for FDA
compliance and identifying and solving breaches in compliance.
From concept through final approval, Groupe Athena navigates companies
through every requirement of the process including pharmaceutical
consulting, development, and regulatory compliance to achieve FDA approval
and export pharmaceuticals and medical products and equipment to the USA.

Services Offered:
Analytical QC
QA Functions
Readiness for Inspections
Compliance
Validation
Clinical Trial Management
Regulatory/Legal
Preparation of Regulatory Filings (IND's/PMA's/NDA's/ANDA's/510Ks etc)
Compliance Assessments (GLP, GMP, GXP)
Risk Assessments
Mock FDA Audits
Medical Device Support
CMC Support
GMP Training
Facility Layout Consulting
Utilities, Process, IT, Computer, and Cleaning Validation
Training

Case Studies
Case Study 1:
A medical device manufacturer had a failing QC testing group though group
facilities in Europe had a higher rate of productivity and much higher rate
of efficiency in terms of fewer laboratory investigations and samples
analyzed.
The project was to analyze the situation and produce a new laboratory
solution to establish at least parity with the European laboratory.
The Process:
Both laboratories were assessed using PERT/CPM tools and the results were
process mapped to discover the differences between the laboratories.
Following this process the analytical laboratories were reviewed for
similarities and an optimum process was developed for the process going
forward.
With the optimum process in place a gap analysis was developed and a forward
implementation plan was crafted. Part of the solution was a total redesign
of the analytical laboratory as well as an upgrade of the operating
practices.
Outcome:
The result of the project was a vastly improved operating situation with
reduced laboratory investigations and an improved level of productivity and
operating efficiency.
Case Study 2:
The Issue
A major specialty pharmaceutical and medical products manufacturer
identified a need to improve inconsistent handling of service and complaint
issues related to their marketed products. From both regulatory and business
liability perspectives, it is critically important to pharmaceutical and
medical device companies that they have robust and compliant drug safety
monitoring processes. The company sought help from Groupe Athena to
standardize and optimize their service and complaint handling process, while
building an operating model and functional organizational structure to
support their future growth objectives.
Our Approach
Groupe Athena provided consulting experience to augment the company’s
internal Global Complaint Management team in the area of program support.
The Outcome
Groupe Athena formed an internal team at the client who was responsible for
developing, maintaining, and integrating the program and working group
plans, reporting progress to key decision makers, identifying and requesting
additional resource requirements, and providing first-line quality assurance
for program activities and deliverables.
Groupe Athena supported the team by developing and maintaining project
plans, preparing agendas and updates, developing reference materials,
facilitating and coordinating working sessions, documenting working group
meeting progress and work products, assuring timely issue resolution, and
assisting with the preparation of deliverables.
Case Study 3:
An acquisition of a generic pharmaceutical company by a rival resulted in an
order that required the divestiture of 3 products to a third party acquirer.
The responsibility of Groupe Athena was to act as the overseer for the
divestiture to review: (1) the transfer of product, technology and marketing
information, (2) the implementation of the technology by the acquiring
company and (3) the subsequent verification that the client did not have
access to the Confidential Business Information.
Website:
www.groupeathena.com
-
GROUPE ATHENA, INC. CONTINUES PACE FOR RECORD FISCAL YEAR WITH STRONG SECOND QUARTER SALES OTC Markets(Sat,
Feb 15)
-
GROUPE ATHENA, INC. FinancialsEDGAR
Online Financials(Sat,
Feb 15)
-
Groupe Athena, Inc. Sets Pace for Record Fiscal Year With 22%
Increase in First Quarter RevenuesMarketwired(Mon,
Oct 21)
-
Groupe Athena, Inc. Announces Revenue Update, Projects $65 Million
in RevenuesMarketwired(Mon,
Sep 23)
-
OtcbbJournal.com, Providing Savvy Traders With Their Micro-Cap Stock
ResearchMarketwired(Tue,
Sep 10)
-
Groupe Athena, Inc. Welcomes New Director, Announces Intent to Apply
for UK LicenseBusiness
Wire(Thu,
Sep 5)
-
Groupe Athena, Inc. Welcomes New Director, Announces Intent to Apply
For UK LicenseAccesswire(Tue,
Sep 3)
-
Groupe Athena Inc. Releases Year End Financials, Continues Record
Revenue and Earnings GrowthAccesswire(Mon,
Aug 5)
-
Groupe Athena, Inc. Releases 3rd Quarter Financials, Continues
Strong Revenue And Earnings GrowthPR
Newswire(Wed,
Apr 10)
-
Groupe Athena, Inc. Enters Multi Year Contractual Relationship With
Major Bangladesh Pharmaceutical ManufacturerPR
Newswire(Mon,
Mar 4)
Information compiled from public sourcess
|

