NeoStem Inc.

Investment Highlights
Divested ownership interest in Suzhou Erye
Pharmaceutical Co, increasing its cash position by $12,280,000 - Already
received $1,228,000 (10%)
Acute Myocardial Infarction (AMI)
therapeutic product development team enrolled the first patient in the
PreSERVE Phase 2 clinical trial
Our Progenitor Cell Therapy (PCT) CDMO
service business continues to grow and has added new clients in later stage
clinical trials setting the stage for expansion into larger and
substantially more lucrative commercial manufacturing contracts
Jonathan Sackner-Bernstein, MD, FACC
joined the Company. He was Associate Center Director for Technology and
Innovation at the U.S. Food and Drug Administration's Center for Devices
and Radiological Health
Actively pursuing additional strategic
relationships with major pharmaceutical and biotechnology companies in 2012
WBB Securities LLC - UPGRADED TO STRONG
BUY - $2.00 12 MONTH TARGET
Symbol is NBS
News Link:
http://finance.yahoo.com/q?s=nbs&ql=1
NeoStem Fact Sheet Quick Recap - see below or
Full FactSheet PDF link
hereAug 15, 2012
Fast Facts
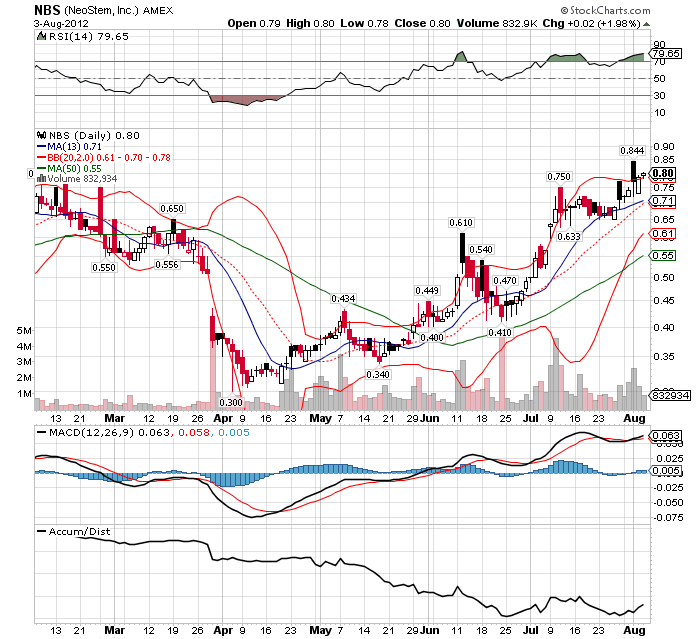
Name: NeoStem, Inc.
Industry: Biotech / Cell Therapy
Ticker (Exchange): NBS (NYSE MKT)
All figures as of 6/18/12 except where noted
Stock Price: $0.47
52 Week Range: $0.30 – 1.76
Market Cap: $62.99 million
Average Volume (3 mos): 1,079,033
Shares Outstanding: 129.9 million*
*As of March 31, 2012
NeoStem Subsidiaries

Established Leader in Cell Therapy Services
150 years of combined management
expertise in manufacturing, regulatory and commercialization for
therapeutics development
$8-10 million in annual historic revenues
Manufactured 30,000+ cell therapy product procedures and delivered
6,000+ cell therapies to patients worldwide for more than 100 clients
50,000 square feet of cGMP manufacturing capability located in North
America (East Coast and West Coast)
Large scale manufacturing for clients allows lower costs for internal
cell therapy development 
•
Establish early partnering relationships with goals of commercial
manufacturing, equity participation and back-end royalties

Emerging Leader in Cardiovascular Cell Therapy
Lead product, AMR-001, an autologous
adult stem cell therapy for the prevention of major adverse cardiac
events following acute myocardial infarction (AMI)
Launched Phase 2 clinical trial in 1Q-2012, data readouts expected
2H-2013
Potential for multiple indications beyond STEMI (i.e., congestive heart
failure, other related vascular insufficiencies)
|
PreSERVE AMI Trial Phase 2 Clinical Plan |
|
Indication |
Post-AMI preservation of
cardiac function |
|
Number of Subjects, Sites |
160 patients, 34+ sites |
|
Primary Endpoint |
Increased cardiac
perfusion |
Amorcyte: Using The CD34+ Natural Repair Mechanism
The body
attempts to rescue damaged tissue to prevent ventricular remodeling:
A distress signal (HIF) is induced by hypoxia in the peri- infarct zone
HIF induces syntheses of SDF and VEGF, which mobilize CD34+CXCR4+ cells
The mobilized cells are trophic to the peri-infract zone, preventing
apoptosis and effecting neoangiogenesis
AMR-001 is a homogeneous and highly purified cell population enriched
for CD34+CXCR4+ cells

In Partnership with Becton Dickinson (20% Owner)
Immunotherapy Platform
• Immune mediated diseases are
a result of imbalance between T effector cells and T regulatory cells
• T-reg therapy represents a
novel approach for restoring immune balance by enhancing T-reg cell
number/function
Opportunities in Regenerative Medicine
Cellular Differentiation with an Eye Toward Macular
Degeneration
• Very small embryonic-like
stem cells, shown to have several characteristics generally found in
embryonic stem cells
• Activities have received
awards of > $2.5 million to support this work
Upcoming Milestones
• Advance VSELs into a Phase Ia
safety trial for macular degeneration (2013)
• PCT – Secure additional
client contracts, establish client partnerships for commercial
manufacturing and/or royalties, and expand manufacturing outside the
U.S. (2012)
• Closing of divestiture of
Suzhou Erye (51% NeoStem owned generic pharmaceutical company) (2012)
• Start Phase I trial in CHF
with AMR-001 (2012/2013)
• Complete enrollment of Phase
II trial (1H-2013)
• Data readouts for Phase II
AMR-001 Trial (2H-2013)
• Athelos - data readout from
work under independent physician INDs (1H-2013)
• Secure additional SBIR and/or
DoD government grants for VSEL Technology (2012)
Contacts
Collaborations
Dr. Robin L. Smith
Chairman and Chief Executive Officer
212.584.4174
rsmith@neostem.com
Gitanjali Jain Ogawa
Trout Group - Vice President
646.378.2949
gogawa@troutgroup.com
This material contains forward-looking statements
within the meaning of the Private Securities Litigation Reform Act of
1995. Forward-looking statements reflect management’s current
expectations, as of the date hereof, and involve certain risks and
uncertainties. Forward-looking statements include statements herein with
respect to the successful execution of the Company’s business strategy,
including with respect to the development of AMR-001 and other cell
therapeutics, the future of the cell therapeutics industry, and the
Company’s divestiture of its interest in Suzhou Erye Pharmaceutical Co.,
Ltd. The Company’s actual results could differ materially from those
anticipated in these forward- looking statements as a result of various
factors including those described under the heading "Risk Factors" in
the Company’s filings with the Securities and Exchange Commission (www.sec.gov).
The Company’s further development is highly dependent on future medical
and research developments and market acceptance, which is outside its
control.
NeoStem, Inc. (NYSE MKT: NBS)
420 Lexington Avenue, Suite 450
New York, NY 10170
212.584.4180
www.neostem.com
NeoStem, Inc.
420 Lexington Avenue
Suite 450
New York, New York 10170
Telephone: 212-584-4180
Fax: 646-514-7787
Website:
www.neostem.com
-
Conceptus, RTI International Metals Among Stocks Gapping Up
Wednesdayat
Fox Business(Wed,
Aug 1)
-
Costly Mistakes Crush Dendreon But Upside May Lurkat
Seeking Alpha(Wed,
Aug 1)
-
Celsion and Neostem Showing Positive Growth as Biotech Industry
Soaring in 2012Marketwire(Tue,
Jul 31)
-
Are Stem Cells Becoming The Next Bull Market In The Biotech
Space?at
Seeking Alpha(Mon,
Jul 30)
-
NeoStem's Promising VSEL Developments Are Good Signs For The
Future Of Cell Therapyat
Seeking Alpha(Thu,
Jul 26)
-
Heavy Volume May Mean Heavier Profits In Small Biopharmaceutical
Stocksat
Seeking Alpha(Mon,
Jul 23)
-
A Big Win For Neostemat
Seeking Alpha(Thu,
Jul 19)
-
NeoStem Continues To Rally Behind Its Key Developmentsat
Seeking Alpha(Tue,
Jul 17)
-
NeoStem's Subsidiary, Progenitor Cell Therapy, and SOTIO Enter
Into a Phase 3 Manufacturing Services AgreementGlobeNewswire(Mon,
Jul 16)
Information compiled from public sourcess
|

